Press release: Viral gene therapy vectors for the first time without bacterial resistance genes
PlasmidFactory’s Minicircle technology reaches a milestone in the manufacture of AAV
If adeno-associated viruses (AAV) can be used as “gene ferries”, they also have the potential to transfer bacterical resistance genses. In the manufacture of AAV vectors, such resistance genes can be transferred to AAV particles by transient plasmid transfection, and as such can reach the target organism.
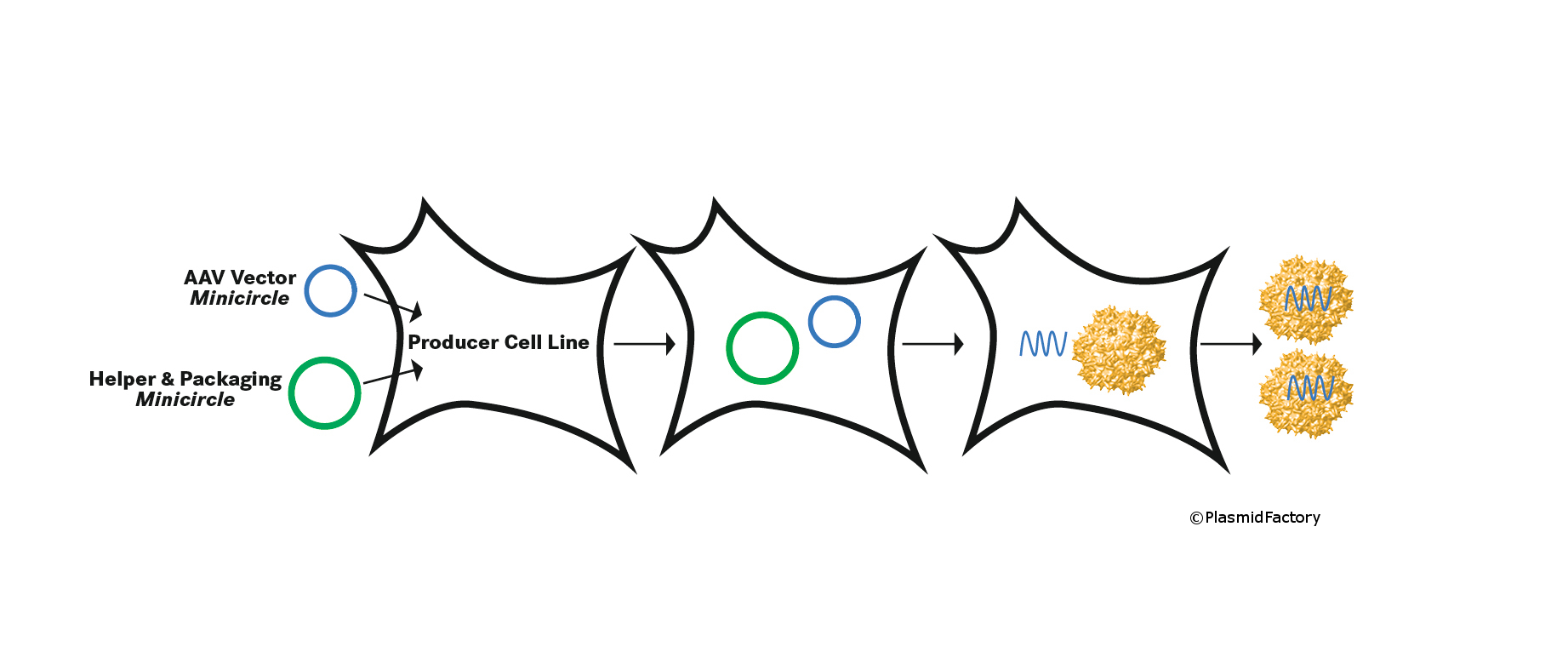
For this set of problems, the company PlasmidFactory in Bielefeld, the Medical University Hanover (MHH), the Centre for Molecular Medicine (ZMMK) at the University of Cologne, as well as the Cornea Laboratory at the University of Erlangen have developed a strategic solution as part of a collaboration, and have thereby reached an important milestone for the future manufacture of therapeutically applicable viral vectors without such contaminations. The researchers used recombinant AAV vectors as a model system. These vectors are derived from adeno-associated viruses and have to date been successful in tests within the framework of gene therapy studies. The cooperation partners were able to show concrete evidence that AAV vectors can be produced based on Minicircle DNA, instead of conventional plasmids, whereby the incorrectly-packaged bacterial sequences described above can be avoided in the AAV capsids. These DNA Minicircles are recombined from so-called parental plasmids to circular derivatives. Subsequently, the prokaryotic plasmid components, which are only required for the replication of constructs in a bacterial culture, are removed from the construct and purified. This results in a monomeric, circular, supercoiled DNA molecule, which almost exclusively contains the gene sequences needed for the actual application in AAV vector production, but not the ones required solely for the replication in E. coli bacteria.
The research work was supported on the part of BMWi with government funds (ZIM Project), which emphasises its importance. The initial results have now been published by the interdisciplinary research team in Molecular Therapeutics: Nucleic Acids, doi: 10.1038/mtna.2016.60.
The frequently-used viral vectors are generally produced based on plasmids as the most important original substance. In this way, the plasmids are coded for the therapeutic gene and contain information on the intracellular production and packaging of this gene in the AAV particle. The Bielefeld company has been successfully manufacturing the basic plasmid in a special laboratory for customers all over the world for several years. Currently, more and more AAV vectors are being successfully implemented in clinical studies for the introduction of genetic information into certain target tissues.
Aside from the application as an agent for complementing genetic defects in terms of cell and gene therapy, there are further future clinical applications of plasmid DNA as non-viral or viral vectors, like the AAV vectors discussed here. These applications could include DNA-based immunisation, for example against viruses, pathogenic spores or tumour tissue (genetic immunisation), or the activation of tissue regeneration, for example for cardiovascular diseases (angiogenesis).
PlasmidFactory holds an exclusive global licence for the manufacture and application for the Helper & Packaging plasmids of the pDG/pDP family by DKFZ Heidelberg, which are used in the production of AAV vectors. These plasmids enable simple and safe production of AAV vectors of different serotypes with high titres. Additionally, PlasmidFactory owns the essential rights to Minicircle technologies worldwide.

